SDS – New requirements 2023
Amendments and modifications to the REACH and CLP regulation are gradually introducing increased requirements for the format and content of the SDS. It requires companies to plan an implementation process to ensure compliance with legislative requirements.
SDS changes:
Each supplier of chemical mixtures must fulfil obligations under Article 31 of REACH (Requirements for safety data sheets) and Annex II of REACH regulation. The references to the legal text have been updated to reflect the latest version of Annex II (i.e. the Annex to Regulation (EU) 2020/878).
The Commission Regulation (EU) 2020/878 introduces changes in the scope of: new requirements for nanoforms of substances, adapting to the 6th and 7th revision of the GHS, and adding requirements regarding the Unique Formula Identifier (as set by Annex VIII to Regulation (EC) 1272/2008), endocrine disrupting properties, specific concentration limits, M-factors and acute toxicity estimates.
The document provides for changes in the provisions and the format of the safety data sheet. The most important changes include:
- new format for section 9
- separation of a new subsection 11.2
- separation of new subsections 12.6 and 12.7
- the wording of the subsection has been changed in sections 14.1 and 14.7
Transitional period:
New requirements for Safety Data Sheet shall apply from 1 January 2021, but in accordance with Article 2 of Regulation (EU) 2020/878, safety data sheets compiled in accordance with Regulation (EC) No 1907/2006 as amended by Commission Regulation (EU) 2015/830, can continue to be used until 31 December 2022. All safety data sheets provided after 31 December 2022 have to be in the format according to Regulation (EU) 2020/878.
This is without prejudice to the obligation to update the safety data sheets in accordance with Article 31(9) of Regulation (EC) No 1907/2006, and to the cases where the Unique Formula Identifier (UFI) is added to safety data sheets as provided for in section 5 of Part A of Annex VIII to Regulation (EC) No 1272/2008 (CLP). https://ekotox.de/news/ufi-codes-new-duties-from-january-2021/
Transitional period including the following scenarios/actions:
- No change to safety data sheet
- Small change to safety data sheets not within scope of Article 31(9)
- Update to safety data sheets within the scope of Article 31(9) or introducing the UFI
- New safety data sheets authored for the first time after 1st January 2021
New hazard classes 2023
Application dates
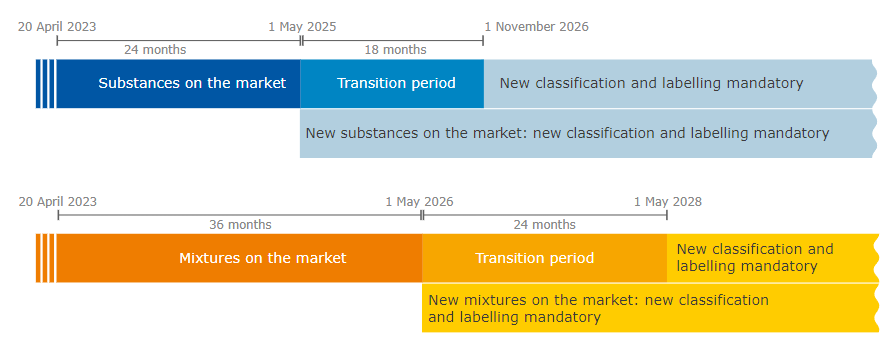
The new rules are in force as of 20 April 2023. From this day on, the Member States can make proposals for harmonised classification and labelling (CLH) with the new hazard classes and manufacturers, importers, downstream users and distributors can self-classify their substances and mixtures accordingly.
There are transitional periods from the entry into force of the Delegated Regulation, during which manufacturers, importers, downstream users and distributors are not yet required to classify their substances or mixtures according to the new hazard classes. During these periods, the new hazard classes can be applied on a voluntary basis.
At the end of the transitional periods, all manufacturers, importers, downstream users and distributors must apply the new hazard classes.
How to prepare for changes:
WHO? – Verification of the company’s profile and position in the supply chain.
Where there is a chain of supply, the requirements of REACH in relation to the provision of safety data sheets apply at each stage of the supply chain. Each entity from the chemical industry: producers, suppliers of substances, mixtures, products for professional use or finished products, especially detergents and biocides, as well as professional workers, shops and retailers have to ensure that they are required to prepare, share and keep safety data sheets.
WHAT? – Identification of all substances, mixtures and products produced, supplied and used in the company.
The supplier of a substance or a preparation shall provide the recipient of the substance or preparation with a safety data sheet compiled in accordance with Annex II:
(a) where a substance or preparation meets the criteria for classification as dangerous in accordance with Directives 67/548/EEC or 1999/45/EC; or
(b) where a substance is persistent, bioaccumulative and toxic or very persistent and very bioaccumulative in accordance with the criteria set out in Annex XIII; or
(c) where a substance is included in the list established in accordance with Article 59(1) for reasons other than those referred to in points (a) and (b).
HOW? – Analysis of possessed documents and resources.
Start with a well-designed action plan to implement the legislative requirements efficiently. A typical action plan includes the following steps:
STEP 1: Start with Ekotox REACH + SDS Screening – it allows you to identify your company’s profile, identify all chemical substances, mixtures and products, prepare list of chemicals needed SDS and identify gaps in your chemical documentation.
STEP 2: Estimate the time needed for changes and determine the total cost. Prepare a strategy for adapting to change, taking into account the possibilities offered by the transition period: whether the changes will be made in full or in part.
STEP 3: Contact suppliers and partners to get all information needed to meet the obligations resulting from new legal changes. Collect the registration numbers of the substances, the current toxicological data of the substances as well as the DNEL and PNEC values, endocrine disrupting properties etc.
STEP 4: Update your chemical documentation and prepare new Safety Data Sheets in the format according to Regulation (EU) 2020/878, in the required official languages of the Member States where the product is placed on the market. Keep in mind that the safety data sheet must be prepared by a competent person. Ekotox Centers provides you with SDS consultancy and the service of preparing safety data sheets in many languages. We offer a discount on the entire volume of your SDS. https://ekotox.de/about-company/market-surveilance/
STEP 5: Send new SDSs to your recipients before the deadline and make sure that the documents you keep conform to the new format.
WHEN? – Setting the schedule and priorities.
There is no one-size-fits-all solution for every company. It depends on the amount of products that the company have in portfolio and that are subject to REACH requirements. If the company is a manufacturer and has a complete dossier for each substance, the implementation of the changes will be faster and easier. If the company is an importer or downstream user must cooperate with other entities. For non-EU suppliers it will be more difficult and time consuming to obtain the required information and substance dossier. In time it should be considered whether there are no other issues for updating SDS before 31/12/2022, e.g. substance reclassification, updating toxicological information, etc. https://ekotox.de/update-of-safety-data-sheet/