REACH-Zulassung
Die Ökotox-Zentren bieten Beratungsdienste mit Schwerpunkt auf der REACH-Gesetzgebung, dem Management von SVHC-Stoffen, der Kommunikation in der Lieferkette und der Unterstützung bei der Einhaltung der EU-Chemikaliengesetzgebung.Wir haben mehrere Projekte in Bezug auf die folgenden Themen durchgeführt:
- REACH-Zulassungsanträge – Entwicklung der gesamten REACH-Zulassungsdokumentation.
- Einhaltung der Vorschriften für nachgeschaltete Anwender – Interne Dokumentation für die Einhaltung der REACH-Zulassung (IDRAC).
Wenn Sie Fragen haben, kontaktieren Sie uns bitte:
Telefon +421 2 45943712 / E-Mail ekotox(at)ekotox.eu
FB: Ekotox Centers
Twitter: Ekotox Centers
Linkedin: Ekotox
REACH-Zulassungsdienste:
- REACH-Zulassungsantrag
Vollständige Verwaltung, Koordination und Ausarbeitung des Antrags auf REACH-Zulassung:
- Entwicklung der Antragsstrategie;
- Stoffsicherheitsbericht;
- Analyse von Alternativen;
- Sozioökonomische Analyse;
- Substitutionsplan.
2. REACH-Zulassung/SVHC-Screening/Audit
Unternehmen (Produzenten/Importeure) müssen jetzt den Status der Verwendung von besonders besorgniserregenden Stoffen in ihrem Produktportfolio – Gemische und Erzeugnisse (Artikel), die auf den EU-Markt gelangen – kennen. Das bedeutet auch, dass sie ihre Lieferanten auf die Einhaltung der EU-Chemikaliengesetzgebung überprüfen müssen.
- Bestandsaufnahme der Verwendung von SVHCs und ihres Status;
- Analyse der SVHCs für allgemeine Anforderungen, SCIP und mehr;
- Entwicklung der „Unternehmensstrategie“;
- Entwicklung von internen Regeln und Anwendungshandbüchern;
- Kommunikationsstrategie;
- Plan zur Anwendung von SVHCs.
3. Screening/Audit der Einhaltung der Vorschriften für nachgeschaltete Anwender
Unternehmen (nachgeschaltete Anwender) stehen unter dem Druck der zusätzlichen Anforderungen, den Status der Verwendung von SVHCs zu verstehen. Wenn sie SVHCs in ihrem Produktportfolio haben, besteht das Risiko eines zusätzlichen Verwaltungsaufwands sowie eines Preisanstiegs bei Lieferungen, die von der REACH-Zulassung betroffen sind. Zusätzliche Kosten für die Einhaltung der REACH-Zulassung sowie das Risiko, dass ein solcher Stoff verschwindet, sind eine große Herausforderung.
- Bestandsaufnahme der Verwendung von besonders besorgniserregenden Stoffen und deren Status;
- Verwendung von Anhang XIV-Stoffen und deren Status;
- Entwicklung der – Internen Dokumentation für die Einhaltung der REACH-Zulassung (IDRAC);
- Kommunikations- und Umsetzungsstrategie;
REACH-Zulassungsverfahren
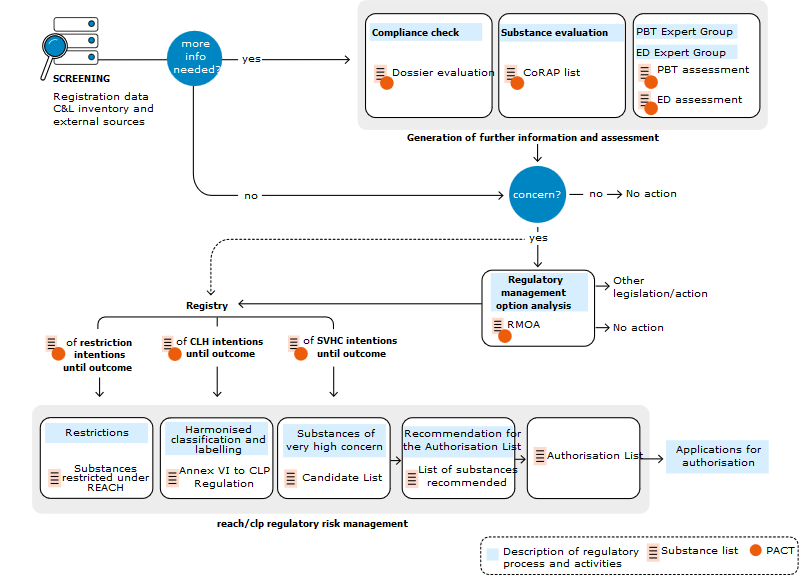
Das Zulassungsverfahren wird in dem unten stehenden Schema beschrieben:
Link zur ECHA: https://echa.europa.eu/de/substances-of-potential-concern
Die REACH-Zulassung bezieht sich auf Stoffe, die in Anhang XIV der REACH-Verordnung – „Zulassungsliste“ – aufgeführt sind.
Sie bedeutet ein vollständiges Verbot der Verwendung von chemischen Stoffen mit Ausnahme der „zugelassenen Verwendung“ – Entscheidung über die Zulassung durch die Europäische Kommission.
Das Zulassungsverfahren erfordert die Erstellung und Einreichung von Unterlagen, die belegen, dass die Verwendung sicher ist oder dass sie derzeit nicht ersetzt werden kann. Für die Einreichung der Unterlagen sind bestimmte Termine festgelegt, ebenso wie der Stichtag für die Antragstellung.
Hersteller, Importeure oder nachgeschaltete Anwender können eine Zulassung für das Inverkehrbringen oder die Verwendung eines in der Zulassungsliste aufgeführten Stoffes beantragen.
Die Zulassung wird erteilt, wenn der Antragsteller nachweisen kann, dass das Risiko, das von der Verwendung des Stoffes ausgeht, angemessen beherrscht wird. Ist dies nicht der Fall, kann eine Zulassung dennoch erteilt werden, wenn nachgewiesen wird, dass der sozioökonomische Nutzen der Verwendung des Stoffes die Risiken überwiegt und es keine geeigneten Alternativstoffe oder -technologien gibt.
Der Antrag umfasst einen Stoffsicherheitsbericht, eine Analyse der möglichen Alternativen und einen Plan zur Substitution des Stoffes, falls geeignete Alternativen verfügbar sind. Er kann auch eine sozioökonomische Analyse enthalten.
Nach Erhalt des Antrags und der Gebührenzahlung erstellen der Ausschuss für Risikobeurteilung (RAC) und der Ausschuss für sozioökonomische Analyse (SEAC) der ECHA ihre Stellungnahmen zu dem Antrag. Sie prüfen zunächst, ob der Antrag den Informationsanforderungen von REACH (Artikel 62) entspricht. Wenn dies nicht der Fall ist, können die Ausschüsse zusätzliche Informationen anfordern.
Die Zulassung ist eine Maßnahme des Risikomanagements für Chemikalien. Ihr Ziel ist es, die Gesundheit und die Umwelt vor Stoffen mit *CMR-, *PBT- und *vPvB-Eigenschaften zu schützen, für die es wissenschaftliche Beweise für wahrscheinliche schwerwiegende Auswirkungen auf die menschliche Gesundheit oder die Umwelt gibt. Diese Stoffe sollten durch weniger gefährliche Stoffe ersetzt werden.
*CMR (krebserzeugend, erbgutverändernd oder fortpflanzungsgefährdend) – Stoffe, die die Kriterien für die Einstufung in Kategorie 1 und 2 gemäß der Richtlinie 67/548/EWG erfüllen.
*PBT (persistent, bioakkumulierbar und toxisch) oder vPvB (sehr persistent und sehr bioakkumulierbar) – Stoffe, die die Kriterien von Anhang XIII der REACH-Verordnung erfüllen.
*Stoffe, für die es wissenschaftliche Belege für wahrscheinliche schwerwiegende Auswirkungen auf die menschliche Gesundheit oder die Umwelt gibt, die ebenso besorgniserregend sind wie die aufgelisteten Stoffe (d. h. Stoffe mit endokrinen Störungen oder solche mit persistenten, bioakkumulierbaren und toxischen Eigenschaften oder sehr persistenten und sehr bioakkumulierbaren Eigenschaften, die die Kriterien in Anhang XIII der REACH-Verordnung nicht erfüllen.
Ein nachgeschalteter Anwender darf einen Stoff nur in Übereinstimmung mit der erteilten Zulassung verwenden, muss den Stoff von dem Unternehmen beziehen, dem die Zulassung erteilt wurde, die Zulassungsbedingungen einhalten und der ECHA die Verwendungen dieses Stoffes mitteilen. Auch ein nachgeschalteter Anwender kann die Erteilung einer Zulassung für seine Verwendung beantragen.
Die Zulassung ist ein relativ langwieriges Verfahren und erfordert genügend Zeit, um über Fragen der Kommunikation, der Zusammenarbeit, des Informationsaustauschs und anderes zu entscheiden.
Bitte kontaktieren Sie uns:
Telefon +421 2 45943712 / E-Mail ekotox(at)ekotox.eu
FB: Ekotox Centers
Twitter: Ekotox Centers
Linkedin: Ekotox
Authorisation: